THYROID CANCER GRAND ROUNDS
Is There New Treatment for Progressive Iodine-Resistant Metastatic Differentiated Thyroid Carcinoma?
Wendy Sacks
Clinical Thyroidology
CASE PRESENTATION • • • • • • • • • • • • • • • • • • • • • • • •
Background
This 68-year-old woman with a history of breast cancer noticed a new neck mass 9 years ago. Ultrasound revealed an irregular-appearing lesion in the left lobe of the thyroid and FNA biopsy demonstrated papillary thyroid cancer (PTC). She underwent thyroidectomy. The tumor was adherent to the surrounding muscle, internal jugular vein, and carotid artery. The pathology demonstrated moderately differentiated classic and follicular variant (FV) PTC that was not encapsulated and essentially replaced the entire left thyroid lobe. The tumor extended into the perithyroidal soft tissue and was present at the inked margins. One central-compartment lymph node was involved without extranodal extension. In addition, there was a 7-mm focus of FV-PTC in the right lobe (pT3N1aMX, stage III). Following a thyroxine-withdrawal protocol, she received 202 mCi of radioactive iodine (RAI). A 7-day scan showed uptake in the anterior neck suggestive of a neoplastic lesion and uptake in the left parotid gland.
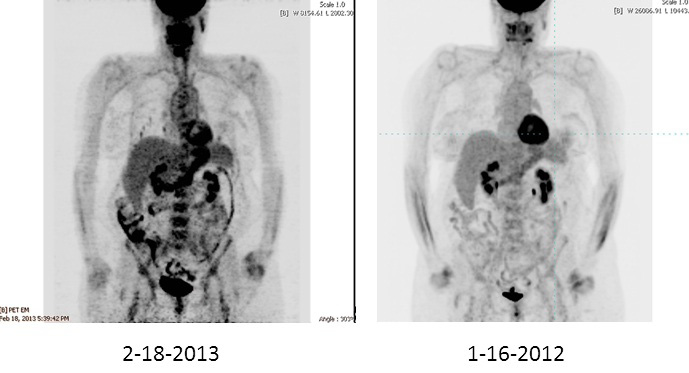 |
| Figure 1. PET scan showing lung nodules in 2013 that were not visible in scan of 2012. |
For the next 5 years, recombinant human TSH (rhTSH) stimulated 131I diagnostic scans showed no uptake and the thyroglobulin (Tg) level was 0.5 ng/ml on thyroid-hormone suppression. Ultrasound of the neck was not done. Five years after her initial surgery and radioactive iodine treatment, a soft-tissue mass was seen in the left neck on ultrasound and FDG uptake in the same area was seen on PET imaging. Her Tg rose from 0.5 ng/ml to 1.9 ng/ml after rhTSH stimulation. Biopsy confirmed recurrent PTC. She underwent excision of this mass. Later that year, she underwent more extensive surgery including central-compartment dissection, left modified neck dissection of levels III and IV, and a partial esophagectomy. Surgical pathology revealed metastatic PTC in the esophageal and paraesophageal soft tissue and in the soft tissue of the left lateral neck (no lymph nodes). She then completed a very complex intensity-modulated radiation therapy (IMRT) plan with 6340 cGy. IMRT uses advanced technology to manipulate beams of radiation to conform to the shape of a tumor, reducing side effects of the treatment. Seven years after the initial diagnosis, CT imaging demonstrated small lung nodules consistent with metastatic disease. On retrospective review of a prior CT scan of the chest that was done when she was first noted to have disease recurrence in the neck, multiple tiny lung nodules (the largest 3 to 4 mm) were seen. At the present time, 9 years after diagnosis of PTC, the lung nodules have increased in size, up to 1.1 cm. PET imaging has demonstrated increased intensity of FDG uptake in several of the lung nodules as compared with the prior 2 years of scans (Figure 1). Lesions less than 7 mm, even if malignant, are difficult to detect using FDG because of the partial volume effect that occurs during reconstruction of the images, making PET less sensitive than CT for detection of small lesions (1) . She is clinically asymptomatic with normal pulmonary-function tests.
ANALYSIS AND COMMENTARY • • • • • •
While the majority of differentiated thyroid cancers have a good prognosis, this patient had several adverse prognostic factors suggesting a poor outcome at her initial diagnosis of PTC, including age over 45 years, residual locoregional disease, and only moderately differentiated PTC histology. She had RAI treatment, and despite a seemingly disease-free period of several years with low Tg levels and a negative diagnostic whole-body scan, her cancer persisted and eventually involved the lungs. Stage IV PTC has a 10-year survival rate of approximately 30%, which falls to 10% if there is progressive disease despite conventional therapy (2). While external-beam treatment can be used to prevent locoregional progression of disease, how do we now treat this patient with dedifferentiated progressive lung metastases? Will she benefit from an empiric dose of RAI? What is the right time to use a targeted therapy?
The therapeutic utility of empiric RAI treatment has been assessed by Sabra et al. who performed a retrospective review of 27 patients with iodine-avid distant metastatic lesions after initial RAI treatment, but negative iodine uptake on follow-up diagnostic scans (3). These patients had persistent structural disease confirmed on imaging. None of the patients had regression of their disease after RAI, and while 44% had stabilization of structural metastases, 56% had progression of disease despite multiple RAI treatment doses. These results imply that the likelihood of successful treatment of our patient’s distant metastases with further RAI is low.
The concept of redifferentiation, or reactivating the RAI uptake function of thyroid carcinoma, remains an ongoing area of research in RAI-refractory thyroid cancer. Several agents have been studied in the past, such as retinoids and thiazolidinediones, but have demonstrated limited benefits (4,5). Lithium has been used as an adjunct to improve iodine retention for ablation, but the benefit is modest (6). In the February 14, 2013, issue of the New England Journal of Medicine, Ho et al. published data showing that the MEK inhibitor, selumetinib demonstrated promising results for improving uptake of RAI in patients with iodine-refractory disease (7) (reviewed in Clinical Thyroidology 2013;25:76-8). Twelve of the 20 patients (60%) treated with selumetinib had 124I uptake that was new or increased or both. All five patients with NRAS mutant tumors had increased iodine uptake, and 4 had partial responses to 131I therapy after pretreatment with selumetinib. In addition, consideration for initiation of a tyrosine kinase inhibitor (TKI) should be made. She is a good candidate for this therapy, considering the progressive disease and good overall performance status; however, since TKIs have limited duration of efficacy, starting one now may be premature.
Conclusions
Thyroid cancer lung metastases eventually developed in this well-appearing, asymptomatic woman with PTC. She may benefit from another empiric dose of RAI, after pretreatment with selumetinib. If possible, she should be enrolled in a clinical trial for treatment with selumetinib, since it is not yet available otherwise.
References
- Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr 1979;3:299-308.
- Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892-9.
- Sabra M, Grewal R, Tala H, Larson SM, Tuttle RM. Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive post-therapy 131I whole-body scans. Thyroid 2012;22:887-83.
- Grüning T, Tiepolt C, Zöphel K, Bredow J, Kropp J, Franke WG. Retinoic acid for redifferentiation of thyroid cancer—does it hold its promise? Eur J Endocrinol 2003;148:395-402.
- Kebebew E, Lindsay S, Clark OH, Woeber KA, Hawkins R, Greenspan FS. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid 2009;19:953-6.
- Koong S, Reynolds J, Movius E, Keenan A, Ain K, Lakshmanan M, Robbins J. Lithium as a potential adjuvant to 131I therapy of metastatic, well differentiated thyroid carcinoma. J Clin Endocrinol Metab 1999;84:912-6.
- Ho A, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623-32.
CLINICAL THYROIDOLOGY • JUNE 2013 VOLUME 25 • ISSUE 6 • © 2013



